MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
-Glucose is treated with an excess of acetic anhydride in the presence of pyridine. Identify the products formed.
(a)
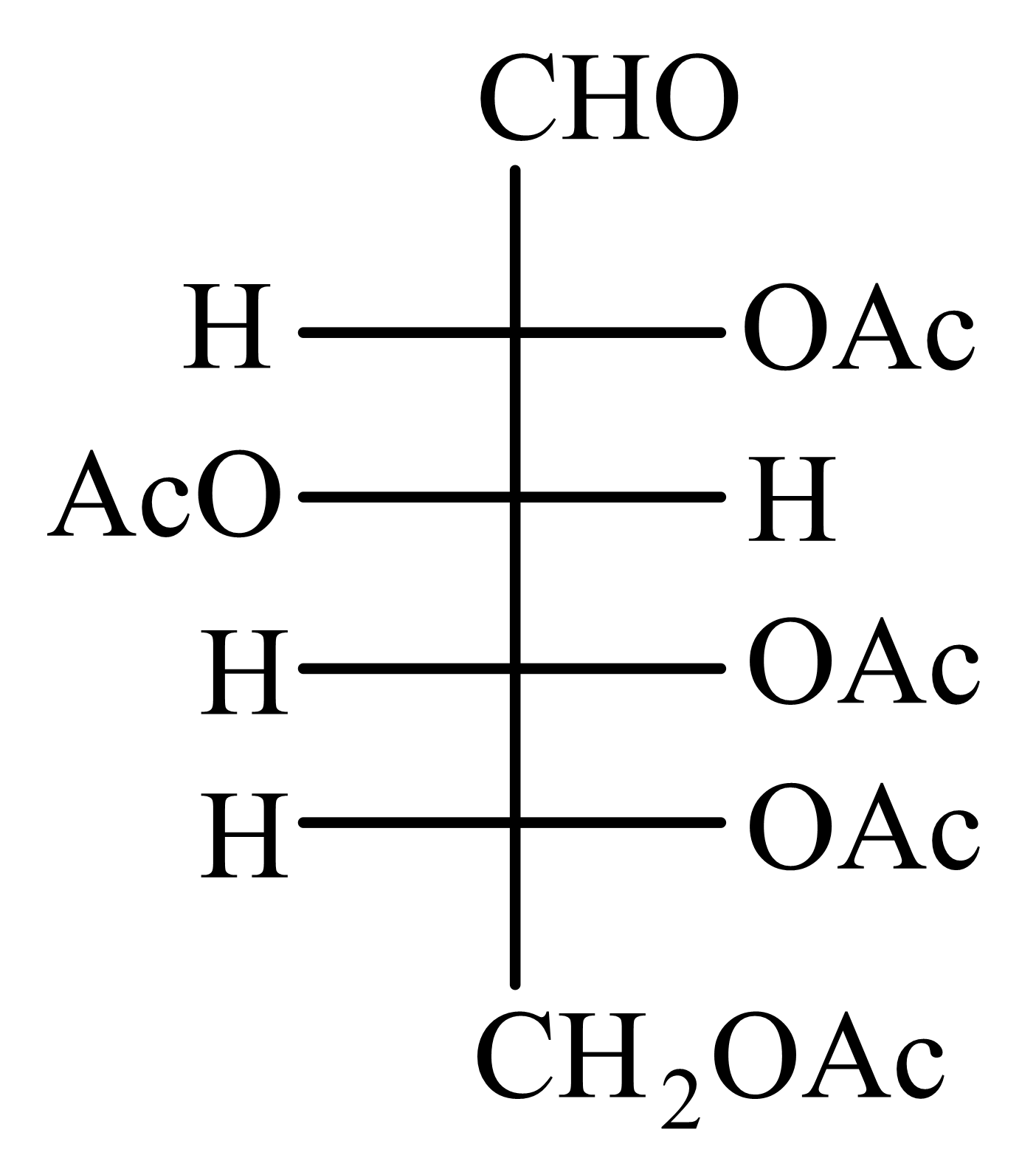
(b)
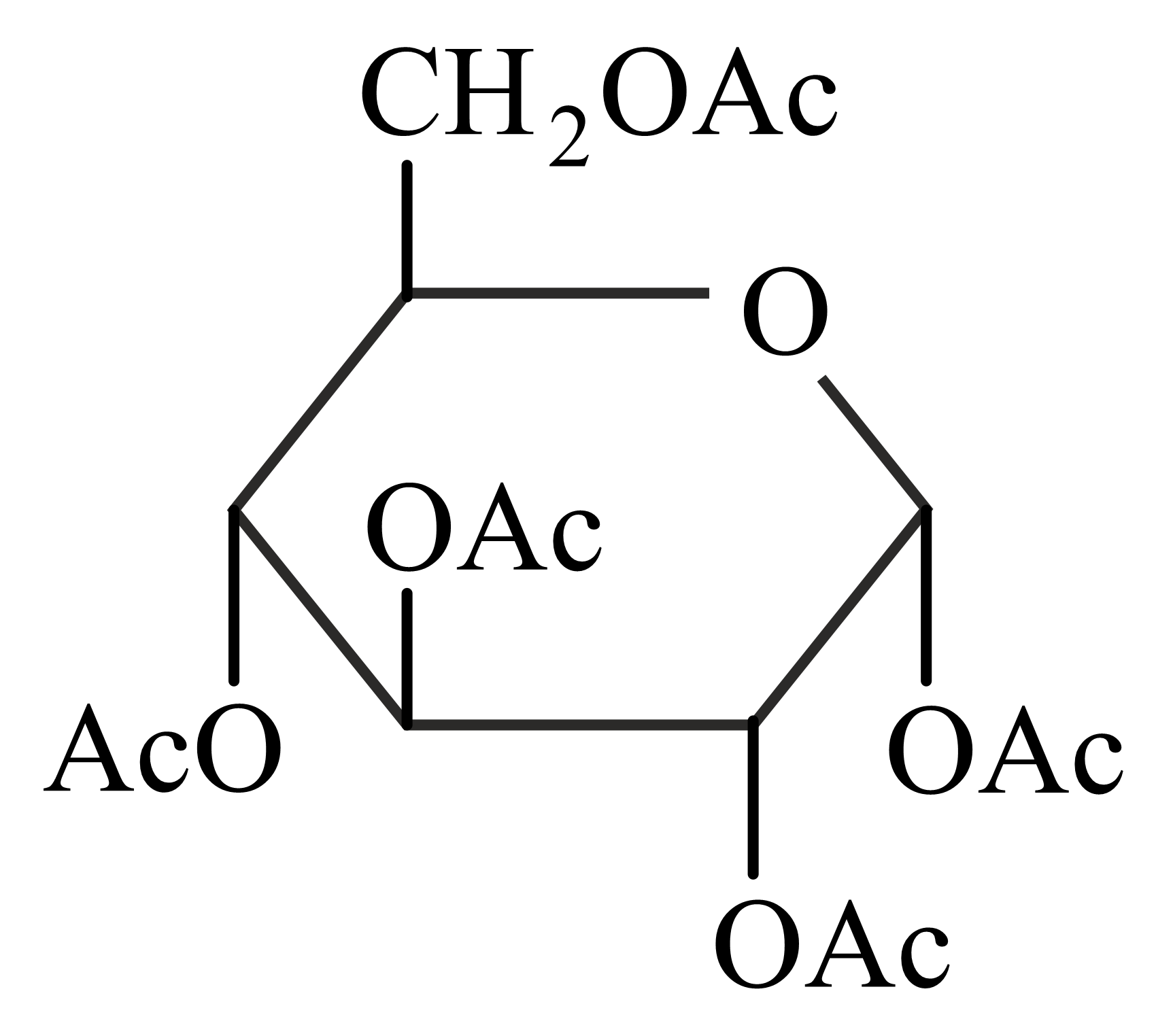
(c)
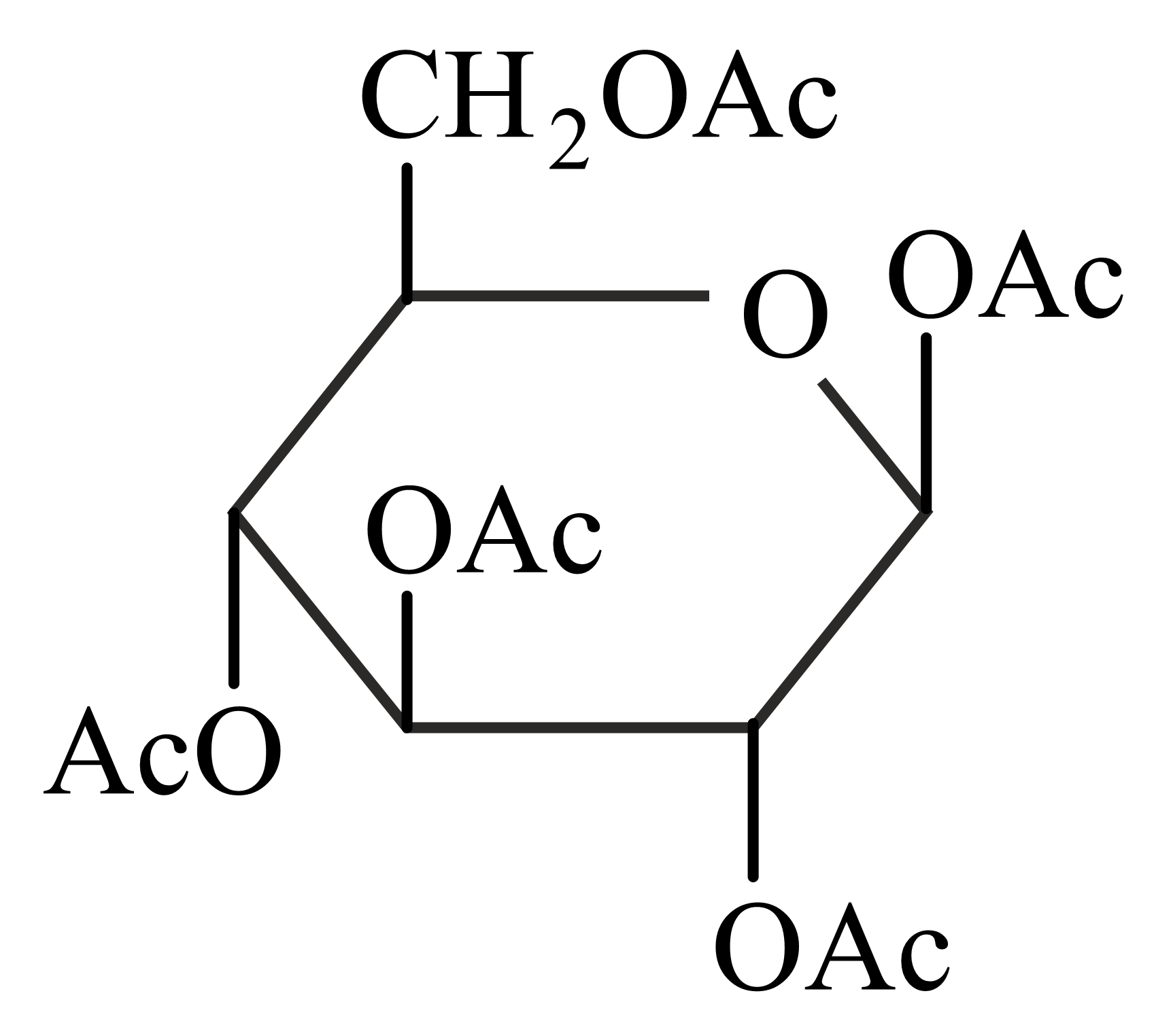
(d)
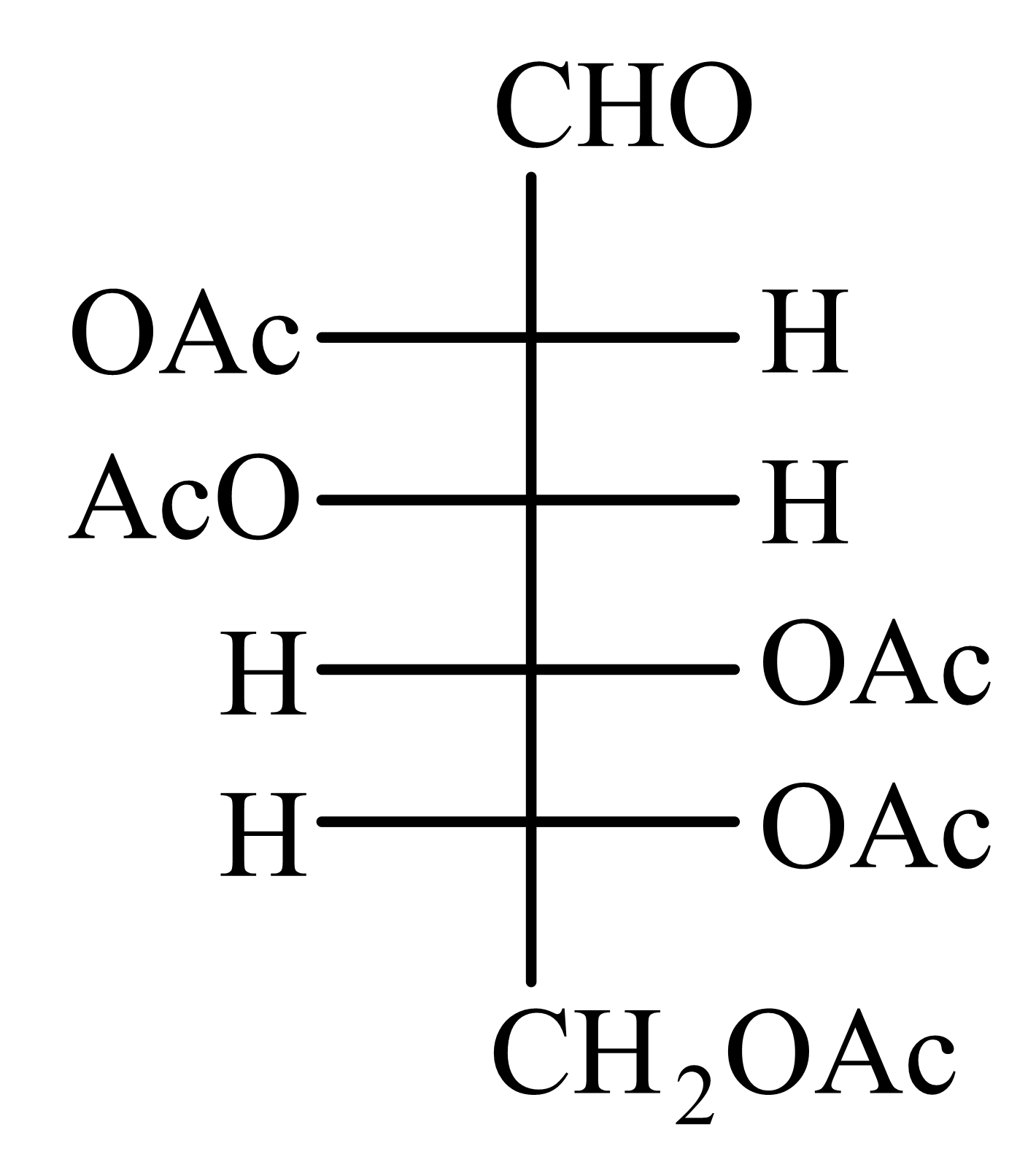
50% studentsanswered this correctly

Important Questions on Biomolecules
MEDIUM
JEE Main/Advance
IMPORTANT
The product formed in the reaction is:
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
