Decarboxylation of all six possible forms of diaminobenzoic acids yields three products and . Three acids give a product '', two acids gives a product '' and one acid give a product ''. The melting point of product '' is

Important Questions on Amines
Identify the major product formed in the following sequence of reactions :
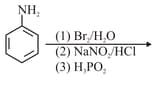
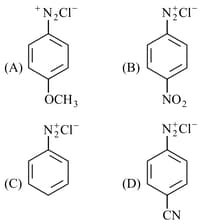
The correct stability order of the following diazonium salt is
Given below are two statements: one is labelled as Assertion and the other is labelled as Reason .
Assertion : Experimental reaction of with aniline and anhydrous does not give and -methylaniline.
Reason : The group of aniline becomes deactivating because of salt formation with anhydrous and hence yields -methyl aniline as the product.
In the light of the above statements, choose the most appropriate answer from the options given below
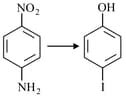
The correct sequential order of the reagents for the given reaction is
Match List-I with List-II
| List-I | List-II | ||
| A | Benzenesulphonyl chloride | I | Test for primary amines |
| B | Hoffmann bromamide reaction | II | Anti Saytzeff |
| C | Carbylamine reaction | III | Hinsberg reagent |
| D | Hoffmann orientation | IV |
Known reaction of Isocyanates. |
Choose the correct answer from the options given below
