MEDIUM
Earn 100
Define dipole moment and explain with an example.
Important Questions on Chemical Bonding and Molecular Structure
EASY
HARD
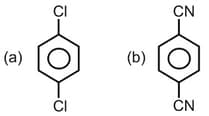
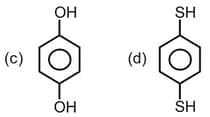
EASY
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
HARD
EASY
EASY
MEDIUM
Find out the correct order of ionic character in the following molecules
EASY
MEDIUM
MEDIUM
EASY
EASY
EASY
EASY
EASY

