MEDIUM
Earn 100
Define duplet set of arrangement of electrons.
Important Questions on Chemical Bonding
MEDIUM
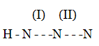
The above compound represents hydrogen azide, the bond orders of bonds and are:
EASY
(A)
(B)
(C)
(D)
(E)
(F)
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
EASY
MEDIUM
EASY

