Define oxidation.
Important Questions on Chemical Reactions and Equations
Explain oxidation and reduction in terms of gain or loss of oxygen. Mention examples for each one.
Explain oxidation and reduction in terms of gain or loss of oxygen. Mention one example for each one.
Answer the following:
In the chemical reaction given below:
Identify the oxidising agent.
Write a balanced chemical equation the following reaction:
Reduction of copper (II) oxide by hydrogen
Answer the following:
In the chemical reaction given below:
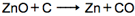
Name the substance which is reduced?
In the above equation, reduction takes place in _____ substance.
Identify the substances that are oxidised and the substance that are reduced in the following reaction:
Identify the substance that is oxidised and the substance that is reduced in the following reaction.
Identify the substance that is oxidised and reduced in the following reaction-
Which reactant is oxidised in the following chemical reaction?
Identify the correct oxidant and reductant in the following reaction
Identify the substances that are oxidised and the substance that are reduced in the following reaction:
Complete the following table which refers to the conversion of ions to neutral particles.
| Conversion | Ionic equation | Oxidation/Reduction |
|
Chloride ion to chlorine molecule |
||
|
Lead ion to lead |
Explain the following in terms of gain or loss of oxygen with one example each:
(i) Oxidation
(ii) Reduction

