MEDIUM
Earn 100
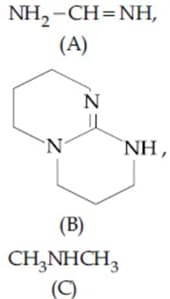
Determine the appropriate order of basicity.
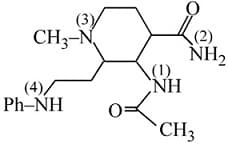

(a)3 > 1 > 2 > 4
(b)3 < 1 < 2 < 4
(c)3 < 4 < 1 < 2
(d)3 > 4 > 1 > 2
50% studentsanswered this correctly
Important Questions on Principles of Organic Chemistry
EASY
HARD
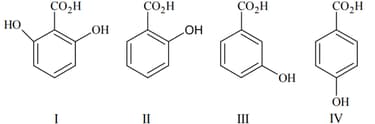
EASY
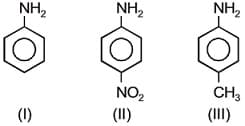
EASY
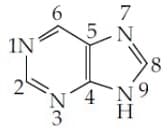
MEDIUM
HARD
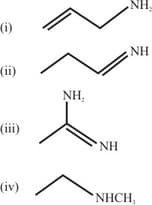
MEDIUM
HARD
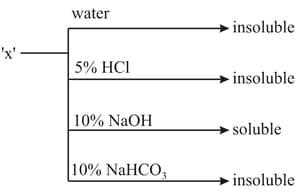
MEDIUM
HARD
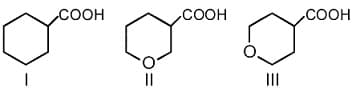
HARD
What is the order of basicity among the following compounds?
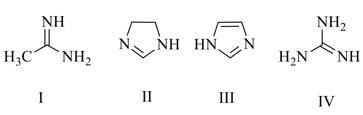
MEDIUM
The increasing basicity order of the following compounds is:
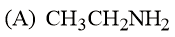
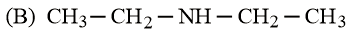
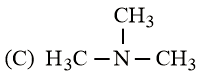
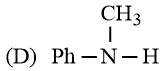
HARD
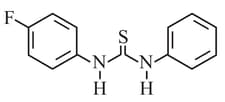
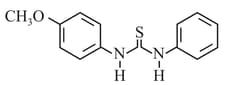
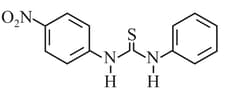
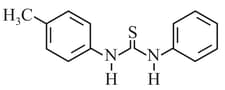
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM

