HARD
Earn 100
Determine the molar solubility of AgCN in water considering hydrolysis of CN- ion give Ksp (AgCN) = 2.3 x 10-16
Ka (HCN) = 5 x 10-10.
(a)1.3 x 10-7
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Equilibrium
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
HARD
HARD
MEDIUM
The stoichiometry and solubility product of a salt with the solubility curve given below is, respectively:
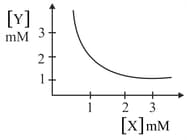
MEDIUM
[Solubility product for ]
HARD
MEDIUM
HARD
MEDIUM
HARD
MEDIUM
MEDIUM
Which of the following choices is correct for a mixture of and
MEDIUM
EASY
MEDIUM
MEDIUM
(Given- the molar mass of )

