Differentiate between physical and chemical equilibrium.
Important Questions on Equilibrium
For this equilibrium, the correct statement(s) is/are
Graph of a reversible process;
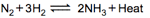
is given. Analyse the graph and answer the following question.
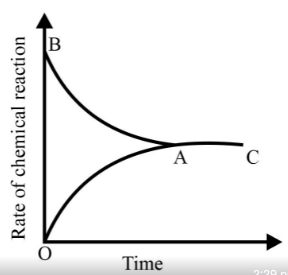
Identify the part of the graph which represents the equilibrium state?
Graph of a reversible process,
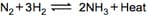
is given. Analyse the graph and answer the following question.
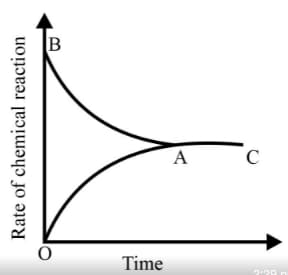
From the given statements, select the correct ones regarding chemical equilibrium.
(i0 The chemical equilibrium is 'static' at the molecular level.
(ii) Both reactants and products co-exist.
(iii) The rates of forward reaction and backward reactions are equal.
(iv) Chemical equilibrium is attained in an open system.
Graph of a reversible process;
is given. Analyse the graph and answer the following question.
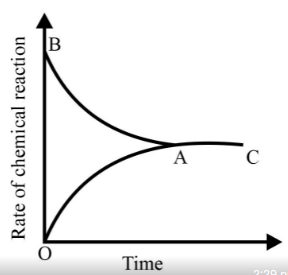
Identify the part of the graph which represents the forward reaction
[ OA, BA, AC]
Which of the following statement is correct about chemical equilibrium?
(i) At equilibrium both the reactants and products coexist.
(ii) At equilibrium rate of forward reaction is greater than the rate of backward reaction.

