EASY
Earn 100
Differentiate heterogeneous system from homogeneous system.
Important Questions on Chemical thermodynamics and energetics
MEDIUM
of ice at is added to 340g of water at . The final temperature of the resultant mixture is . The value of (in g) is closest to
[Heat of fusion of ice ; Specific heat of water ]
MEDIUM
A system consisting of 1 mol of an ideal gas undergoes a reversible process, (schematically indicated in the figure below). If the temperature at the starting point A is 300 K and the work done in the process is 1 L atm, the heat exchanged in the entire process in L atm is
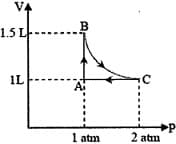
EASY
Calculate the work done during compression of mol of an ideal gas from a volume of to at against a pressure of .
MEDIUM
For the isothermal reversible expansion of an ideal gas
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is




EASY
An ideal gas undergoes isothermal expansion at constant pressure. During the process:
EASY
The work done during combustion of of ethane, at is (Given , atomic mass )
MEDIUM
The molar enthalpy change for at and is . Assuming ideal behaviour, the internal energy change for vaporization of of water at and in is:
HARD
A reversible cyclic process for an ideal gas is shown below. Here, are pressure, volume and temperature, respectively. The thermodynamic parameters are heat, work, enthalpy and internal energy, respectively.

The correct option(s) is (are)
HARD

The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
MEDIUM
A gas is allowed to expand in a well-insulated container against a constant external pressure of from an initial volume of to a final volume of The change in internal energy of the gas in joules will be
MEDIUM
The qualitative sketches I, II and III given below show the variation of surface tension with molar concentration of three different aqueous solutions of KCl, and at room temperature. The correct assignment of the sketches is -

HARD
The volume vs. temperature graph of 1 mole of an ideal gas is given below
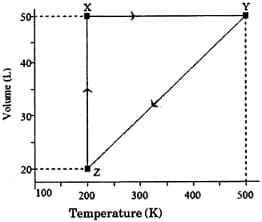
The pressure of the gas (in atm) at and respectively, are
EASY
A gas undergoes change from state to state . In this process, the heat absorbed and work done by the gas is , respectively. Now gas is brought back to by another process during which of heat is evolved. In this reverse process of .
MEDIUM
The three processes in a thermodynamic cycle shown in the figure are : Process is isothermal; Process is isochoric (volume remains constant); Process is adiabatic.
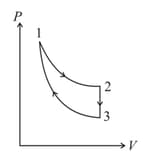
The total work done by the ideal gas in this cycle is, The internal energy decreases by, in the isochoric process. The work done by the gas in the adiabatic process is, . The heat added to the system in the isothermal process is
MEDIUM
An ideal gas in a thermally insulated vessel at internal pressure = , volume = and absolute temperature = expands irreversibly against zero external pressure, as shown in the diagram. The final internal pressure, volume and absolute temperature of the gas are , and , respectively. For this expansion,
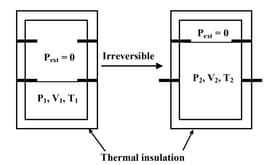
EASY
If of decompose at and , the work is done () by one mole of as it expands against pressure is:
MEDIUM
A piston filled with 0.04 mol of an ideal gas expands reversibly from 50.0 mL to 375 mL at a constant temperature of . As it does so, it absorbs 208 J of heat. The values of q and w for the process will be
(R = 8.314 J/mol K) (ln7.5 = 2.01)
HARD
An ideal gas undergoes isothermal compression from to against a constant external pressure of . The heat released in this process is and is used to increase the pressure of mole of . The temperature of increases by:
EASY
A piece of ice falls from a height so that it melts completely. Only one-quarter of the heat produced is absorbed by the ice and all energy of ice gets converted into heat during its fall. The value of is:
(Latent heat of ice is and )

