HARD
Earn 100
Dissolution of solids in water can be exothermic or endothermic process but gases dissolve in water always with the evolution of heat. Dissolution of a substance in water can be either because ion dipole interactions or by hydrogen bond formation. Pressure plays a significant role on the solubility to gases in water. Solubility of a gas in terms of mol fraction is related to pressure according to the mathematical relation
On the basic of above paragraph answer the following questions.
When a pinch of salt is added to a freshly opened bottle of cocacola or limca, a lot of effervescence occurs with evolution of a colourless gas
(a)The gas evolved is
(b)The gas evolved is
(c)There is no evolution of gas only decomposition of occurs
(d) is evolved due to its displacement from aerated soft drink
50% studentsanswered this correctly
Important Questions on Solutions
EASY
EASY
MEDIUM

The gas with the highest value of Henry's law constant is
MEDIUM
Henry's constant (in kbar) for four gases and in water at is given below :
(density of water at ) This table implies that :
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
EASY
EASY
The oxygen dissolved in water exerts a partial pressure of in the vapour above water. The molar solubility of oxygen in water is ______
(Round off to the Nearest Integer).
[Given : Henry's law constant for Density of water with dissolved oxygen]
EASY
gas is bubbled through water during a soft drink manufacturing process at . If exerts a partial pressure of then of would dissolve in of water. The value of is _______. (Nearest integer)
(Henry's law constant for at is )
MEDIUM
[Henry's law constant for at bar; density of water at ; mass of ; molar mass of water ]
EASY
From the graph, the value of Henry's constant for the solubility of gas in cyclohexane is
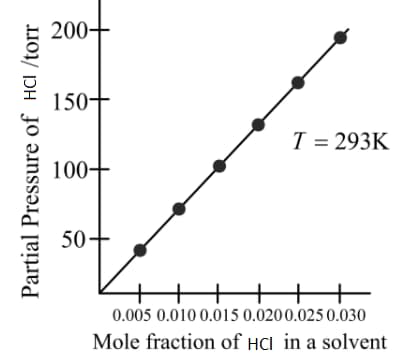
MEDIUM
EASY
HARD

