EASY
AS and A Level
IMPORTANT
Earn 100
Draw a diagram of a hydrogen sulphide molecule to show its shape. Show on your diagram the partial charges on each atom.
Important Questions on Chemical Bonding
EASY
AS and A Level
IMPORTANT
Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years)>Chapter 4 - Chemical Bonding>BEFORE YOU START>Q 1
Explain what is meant by: metallic bonding.EASY
AS and A Level
IMPORTANT
Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years)>Chapter 4 - Chemical Bonding>Questions>Q 9
Answer the following, giving a full explanation in terms of metallic bonding:Explain why aluminium has a higher melting point than sodium.
EASY
AS and A Level
IMPORTANT
Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years)>Chapter 4 - Chemical Bonding>Questions>Q 9
Answer the following, giving a full explanation in terms of metallic bonding:
The thermal conductivity of stainless steel is 82 W m-1 K-1 . The thermal conductivity of copper is 400 W m-1 K-1. Why do some stainless steel saucepans have a copper base?
EASY
AS and A Level
IMPORTANT
Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years)>Chapter 4 - Chemical Bonding>Questions>Q 9
Answer the following, giving a full explanation in terms of metallic bonding:
Why does aluminium conduct electricity better than sodium?
EASY
AS and A Level
IMPORTANT
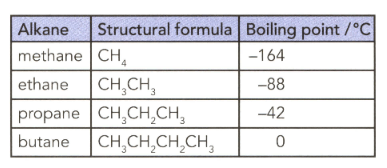
Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years)>Chapter 4 - Chemical Bonding>Questions>Q 11
The table lists the formulae and boiling points of some alkanes. Explain the trend in terms of instantaneous dipole-induced dipole forces.
EASY
AS and A Level
IMPORTANT
Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years)>Chapter 4 - Chemical Bonding>Questions>Q 14
The boiling points of the hydrogen halides are shown in the table.
| Hydrogen halide | HF | HCl | HBr | HI |
| Boiling point / °C | +20 | –85 | –67 | –35 |
Explain the trend in boiling points from to .
EASY
AS and A Level
IMPORTANT
Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years)>Chapter 4 - Chemical Bonding>EXAM-STYLE QUESTIONS>Q 7
Draw a diagram of a hydrogen sulphide molecule to show its shape. Show on your diagram, an arrow showing the exact direction of the dipole in the molecule as a whole.EASY
AS and A Level
IMPORTANT
Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years)>Chapter 4 - Chemical Bonding>EXAM-STYLE QUESTIONS>Q 7
Oxygen, , sulphur, , and selenium, , are in the same group in the periodic table. Explain why hydrogen selenide, , has a higher boiling point than hydrogen sulphide, .