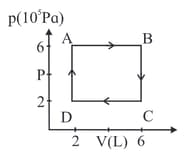
MEDIUM
12th Maharashtra Board
IMPORTANT
Earn 100
Draw a p-V diagram and explain the concept of positive and negative work. Give one example each.

Important Questions on Thermodynamics
EASY
12th Maharashtra Board
IMPORTANT
HARD
12th Maharashtra Board
IMPORTANT
A gas contained in a cylinder fitted with a frictionless piston expands against a constant external pressure of from a volume of
to a volume of . In doing so it absorbs of thermal energy from its surroundings. Determine the change in internal energy of system.
EASY
12th Maharashtra Board
IMPORTANT
MEDIUM
12th Maharashtra Board
IMPORTANT
HARD
12th Maharashtra Board
IMPORTANT
HARD
12th Maharashtra Board
IMPORTANT
A hypothetical thermodynamic cycle is shown in the figure. Calculate the work done in 25 cycles.
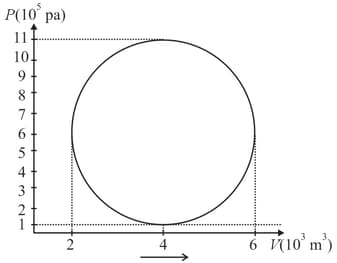
HARD
12th Maharashtra Board
IMPORTANT
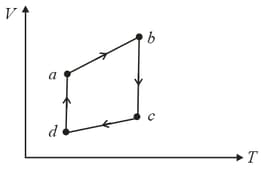
The figure shows the V-T diagram for one cycle of a hypothetical heat engine which uses the ideal gas. Draw the p-V diagram and p-T diagram of the system.
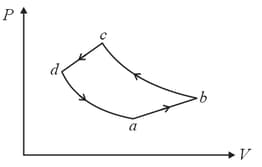
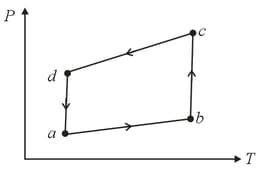
MEDIUM
12th Maharashtra Board
IMPORTANT
A system is taken to its final state from initial state in hypothetical paths as shown figure. Calculate the work done in each case.