HARD
Upper Secondary-IGCSE
IMPORTANT
Earn 100
Draw dot and cross structural diagram to represent the bonding in the following simple molecular compounds. In the dot and cross diagram, show only the outer shells of the atoms involved.
Molecule
Dot and Cross diagram
Structure
Ammonia
Water
Hydrogen chloride
Ethane
Ethene
Ethanol
Ethanoic acid

Important Questions on Elements and Compounds
MEDIUM
Upper Secondary-IGCSE
IMPORTANT
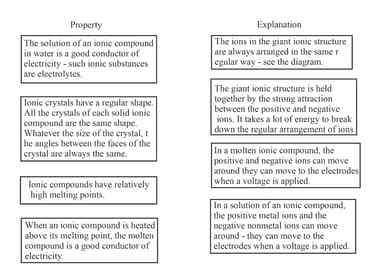
The boxes below contain properties of ionic compounds and their explanations.Draw lines to link each pair.
HARD
Upper Secondary-IGCSE
IMPORTANT
| Observation | Explanation |
| Diamond and silica are both very hard substances _____ . | _____ because all the atoms in the structure are joined by strong covalent bonds. |
| Diamond does not conduct electricity _____ . | _____ because |
| Graphite is _____ . | _____ because the layers in the structure are only held together by weak forces |
| _____ because there are some free electrons that are able to move between the layers to carry the current. |
HARD
Upper Secondary-IGCSE
IMPORTANT
HARD
Upper Secondary-IGCSE
IMPORTANT
HARD
Upper Secondary-IGCSE
IMPORTANT
HARD
Upper Secondary-IGCSE
IMPORTANT
HARD
Upper Secondary-IGCSE
IMPORTANT
