Draw sketch graphs of reaction rate against concentration of the reactant in bold for each of the following reaction:
for which the rate equation is: rate

Important Questions on Reaction Kinetics
Draw sketch graphs of reaction rate against concentration of the reactant in bold for each of the following reaction:
for which the rate equation is:
Note: the catalyst influences the order here – the order is not the same as for the uncatalysed reaction
Draw sketch graphs of reaction rate against concentration of the reactant in bold for each of the following reaction:
for which the rate equation is:
Draw a sketch graph to show how the concentration of the bold reactant changes with time.
for which the rate equation is: rate
Draw a sketch graph to show how the concentration of the bold reactant changes with time.
for which the rate equation is:
Note: the catalyst influences the order here – the order is not the same as for the uncatalysed reaction
Draw a sketch graph to show how the concentration of the bold reactant changes with time.
for which the rate equation is:
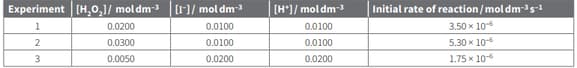
Use the data from experiments 2 and 3 in table to calculate the rate constant for the following reaction.
The rate equation for this reaction is: rate of reaction
Use the formula to calculate a value for the rate constant of a reaction which is first order and has a half-life of .
A first-order reaction has a rate constant of . Calculate a value for the half-life of this reaction.
