Draw the and diagrams for an isobaric process of expansion, corresponding to moles of an ideal gas at a pressure , from to .

Important Questions on Kinetic Theory of Gases
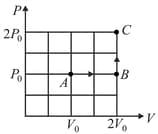
In given diagram, the temperature at is . Find the temperature at point and . Also draw and diagrams for given process.
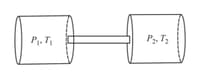
Two identical vessels contain the same gas at pressures and at absolute temperatures and , respectively. On joining the vessels with a small tube as shown in the figure, the gas reaches a common temperature and a common pressure . Determine the ratio .
Aflask of volume litres, provided with a stopcock contains oxygen at and atmospheric pressure. The system is heated to a temperature of , with the stopcock open to the atmosphere. The stopcock is then closed and the flask is then cooled to its original temperature.
(a) What is the final pressure of oxygen in the flask?
(b) How many grams of oxygen remains in the flask?