EASY
Earn 100
Draw the complete formula of methane according to the structural model.
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
Draw the structure of propanal.
MEDIUM
Give the structural formula of Methylpropanol.
EASY
Give the structural formula of acetic acid.
MEDIUM
Write the structural formula of propanone.
MEDIUM
Which of the following structures contain -hybridised carbon atom(s)?
I.
II.
III. 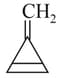
IV. 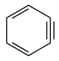
EASY
Write the Fischer structure of Bromobutane.
MEDIUM
Write down the structural formula of methanoic acid.
MEDIUM
Draw the structural formula of butanone.
EASY
In molecule, the hybridization of carbon and respectively, are :
EASY
Which of the following is an incorrect statement?
HARD
Newman projections and are shown below:
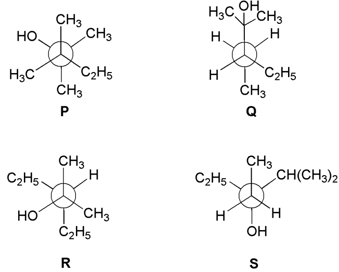
Which one of the following options represent identical molecules?
EASY
The number of C - C sigma bonds in the compound

MEDIUM
The number of hybridised carbons in an acyclic neutral compound with molecular formula is
EASY
How many pi bonds and sigma bonds are present in following molecule?
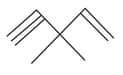
EASY
The number of hybridized carbon atoms in
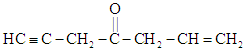
EASY
In the reaction
the hybridization state of the functional carbon changes from
EASY
With a change in hybridization of the carbon bearing the charge, the stability of a carbanion increases in the order
EASY
Which of the following orbital overlaps are involved in the formation of the carbon-carbon single bond in the molecule ?
EASY
The change in the state of hybridization of the asterisks carbon in the following reaction.
is
EASY
The compound butadiene has

