HARD
AS and A Level
IMPORTANT
Earn 100
Draw the displayed formulae and name the structural isomers of .

Important Questions on Introduction to Organic Chemistry
HARD
AS and A Level
IMPORTANT
Draw the displayed formulae and name the functional group isomers of that is:
- an aldehyde
HARD
AS and A Level
IMPORTANT
Draw the displayed formulae and name the functional group isomers of that is:
- a ketone
HARD
AS and A Level
IMPORTANT
Draw the displayed formula and name an isomer of that could be used as an example of the positional isomerism of one of the isomers in the given figures.
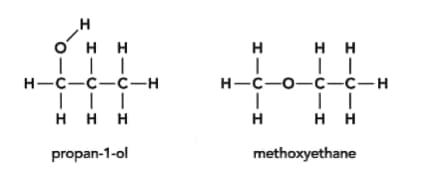
HARD
AS and A Level
IMPORTANT
Draw the displayed formula and give the names of the isomers of .
HARD
AS and A Level
IMPORTANT
Draw the displayed formulae and label the cis / trans isomers of but--ene.
HARD
AS and A Level
IMPORTANT
Draw the cis / trans isomers of -bromo--chlorocyclobutane.
HARD
AS and A Level
IMPORTANT
The molecule exhibits optical isomerism. Draw the displayed formulae of both optical isomers.
HARD
AS and A Level
IMPORTANT
Which one of the following can have optical isomers? Draw the displayed formula of your chosen answer and clearly label its chiral centre.
