MEDIUM
MYP:4-5
IMPORTANT
Earn 100
Draw the isomers of pentane, hexane and heptane.

Important Questions on Does Organic Chemistry Mean We Can Make Any Substance We Want?
EASY
MYP:4-5
IMPORTANT
Determine the mathematical relationship between the number of carbon atoms and the number of isomers in single bonded structural isomers.
MEDIUM
MYP:4-5
IMPORTANT
Determine the mathematical relationship between the number of carbon atoms and the number of isomer in a situation where the effect of one double or triple bond in an unsaturated carbon chain or carbons long.
EASY
MYP:4-5
IMPORTANT
Suggest how you could manufacture an ingredient to make sure that its stereoisomer property is compatible with human consumption.
MEDIUM
MYP:4-5
IMPORTANT
Identify the structural formula, name and unit formula of each of the polymers in the table given below:
| Name of monomer | Structural formula of monomer | Name of polymer | Formula of repeating unit |
| Ethene | 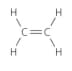 |
Polyethene | 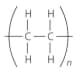 |
| Propene | |||
| Chloroethene(Vinyl chloride) | |||
| Phenylethene(Styrene) | |||
| Tetrafluoroethene | |||
| prop--enenitrile | |||
| Methyl--methylpropenoate (methyl methacrylate) |
MEDIUM
MYP:4-5
IMPORTANT
Explain how a particular organic compound was created in order to address a specific problem or issue.
EASY
MYP:4-5
IMPORTANT
State the name of the functional group in the following compound:
EASY
MYP:4-5
IMPORTANT
State the name of the functional group in the compound .
EASY
MYP:4-5
IMPORTANT
State the name of the functional group in the compound .
