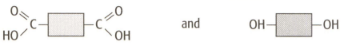Draw the structure of poly(ethene) showing at least two repeat units.

Important Questions on Petrochemicals and Polymers
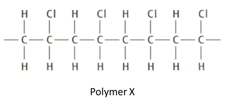
The structure given below is that of an addition polymerisation.
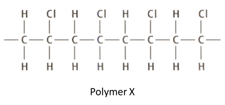
Draw the structure of the monomer from which polymer is formed.
Polymer is non-biodegradable. Describe one pollution problem that this causes.
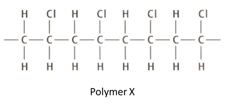
Polymer can be disposed of by burning at high temperatures. However, this can produce toxic waste gases such as hydrogen chloride. Hydrogen chloride can be removed form the waste gases by the reaction with moist calcium carbonate powder. Name the three products of this reaction.
Compare the structure of a protein with that of a synthetic polyamide. The structure of a typical protein is given below:
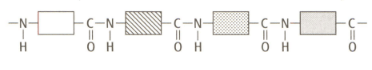
i) How are they similar?
ii) How are they different?
A form of nylon can be made from the following two monomers.

Deduce the simple molecule released in the condensation reaction in this case?
Complex carbohydrates such as starch are another group of condensation polymers. Enzymes such as amylase, a carbohydrase, can hydrolyse complex carbohydrates to simple sugars which can be represented as:
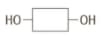
Draw the structure of the complex carbohydrate chain (showing at least three monomer units).
Complete the following table on the third type of important condensation polymer.
| Monomers used |
|
| Structure of the polymer formed | |
| What other product forms | name:_____ formula:_____ |
|
Type of polymer formed (circle one of these possible answers) |
polyamide polyester polysaccharide |
| A name for one polymer of this type | |
| Is the polymer synthetic or natural? |