HARD
Earn 100
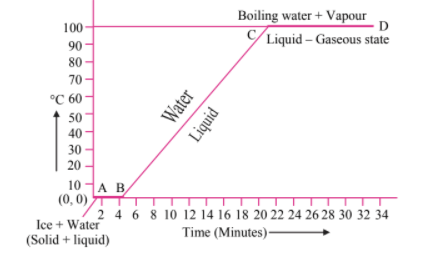
Draw the temperature versus heat graph for heating of ice. Describe the phase change of the water on the basis of the graph. Explain the terms specific latent heat of fusion and specific latent heat of vaporization.
Important Questions on Heat
MEDIUM
[Take specific heat of water and latent heat of steam
EASY
A steam engine intakes of steam at per minute and cools it down to . If latent heat of vaporization of steam is , then the heat rejected by the steam engine per minute is _____
(Given : specific heat capacity of water : )
EASY
MEDIUM
HARD
EASY
EASY
EASY
EASY
Heat required to melt of ice is . A man melts of ice by chewing in one minute. His power is______
EASY
MEDIUM
[ Specific heat of water Latent heat of water ]
EASY
EASY
(Latent heat of ice is and )
MEDIUM
HARD
A thermally insulated cubical box of side length and wall thickness containing of ice is closed on all sides. The mass of ice melted in hours is (Thermal conductivity of the material of the box latent heat of ice and ambient temperature )
MEDIUM
MEDIUM
MEDIUM
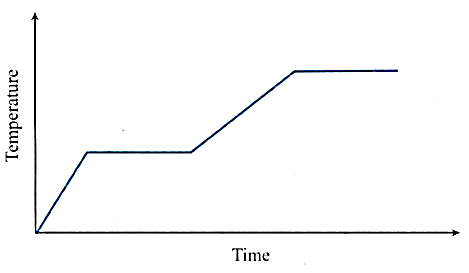
Which of the following conclusions can be drawn?
EASY