EASY
Earn 100
Draw the three-dimensional structure of silicon dioxide.
Important Questions on Chemical Bonding and Structure
MEDIUM
EASY
What would be the products for the following reactions respectively? (water molecules would be the expected biproduct)
EASY
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): In expensive scientific instruments, silica gel is kept in watch-glasses or in semipermeable membrane bags.
Reason (R): Silica gel adsorbs moisture from air via adsorption, thus protects the instrument from water corrosion (rusting) and / or prevents malfunctioning.
In the light of the above statements, choose the correct answer from the options given below:
HARD
EASY
EASY
Explain the structure of silica. How does it react with a. and b. .
MEDIUM
In the above reaction, the compound contains
MEDIUM
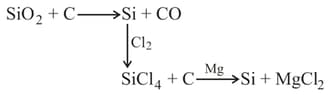
Identify true statement:
MEDIUM
EASY
MEDIUM
MEDIUM
HARD
MEDIUM
EASY
EASY

