During electrolysis of CuSO4(aq) using platinum electrodes, the pH of solution (electrolyte)
Important Questions on Redox Reactions
An aqueous solution of nickel (II) sulphate was electrolyzed using nickel electrodes. Observe the diagram and answer the question that follows:
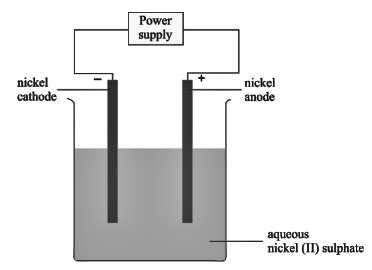
- Which equation for the reaction at the anode is correct?
[Given : ]
(i)
(ii)
(iii)
(iv)
An aqueous solution of nickel (II) sulphate was electrolyzed using nickel electrodes. Observe the diagram and answer the question that follows:
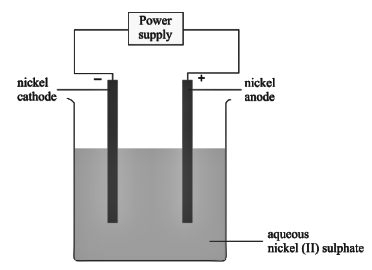
- Name the cation that remains as a spectator ion in the solution.
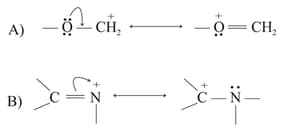
In the following resonance structures, the curved arrow indicates that electrons are shifted from
An aqueous solution of nickel (II) sulphate was electrolyzed using nickel electrodes. Observe the diagram and answer the question that follows:
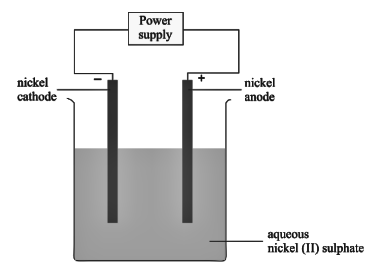
- What do you observe at the cathode and anode respectively?
The electrode potential, for the reduction of to in acidic medium is
V. Which of the following metal(s) will be oxidised? The reduction reactions and standard electrode potentials for and are given as
Given
and
Aqueous is unstable in solution and undergoes simultaneous oxidation and reduction according to the reaction
.
Choose the correct for the above reaction.

