Each atom of mass of a monoatomic gas has got three degrees of freedom. The velocity of these atoms is at temperature For a diatomic molecule of mass and temperature , which has got five degrees of freedom, rms velocity of molecule is,

Important Questions on Thermometry, Thermal Expansion and Kinetic Theory of Gases
A ring shaped tube contains two ideal gases with equal masses and relative molar masses and . The gases are separated by one fixed partition and another movable stopper , which can move freely without friction inside the ring.
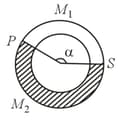
The angle in equilibrium as shown in the figure (in degrees) is
A cylindrical tube of uniform cross-sectional area is fitted with two air tight frictionless pistons.
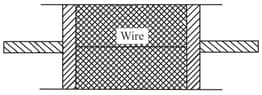
The pistons are connected to each other by a metallic wire. Initially, the pressure of the gas is and temperature is , the atmospheric pressure is also . Now, the temperature of the gas is increased to the tension in the wire will be,
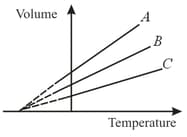
The expansion of an ideal gas of mass at a constant pressure is given by the straight line . Then, the expansion of the same ideal gas of mass at a pressure is given by the straight line.
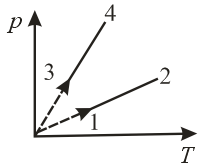
Pressure versus temperature graph of an ideal gas of equal number of moles of different volumes are plotted as shown in figure. Choose the correct alternative.
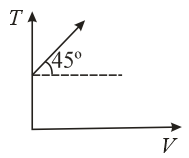
The given curve represents the variations of temperature as a function of volume for one mole of an ideal gas. Which of the following curves best represent the variation of pressure as a function of volume?
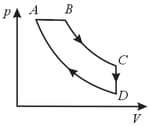
A cyclic process is shown in the diagram. Which of the following curves represent the same process?