Enthalpy () is equal to
Important Questions on Thermodynamics
The internal energy change (in ) when of water undergoes complete evaporation at is ..............
(Given : for water at , )
For which of the following systems, the difference between and is not significant?
(i) Solids
(ii) Gases
(iii) Mixture of gases and liquids
(iv) Liquids
The combination of plots which does not represent isothermal expansion of an ideal gas is
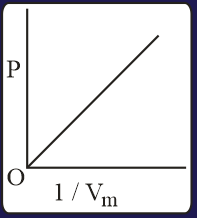
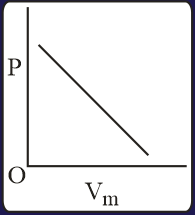
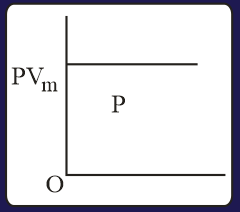
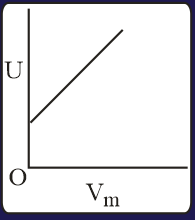
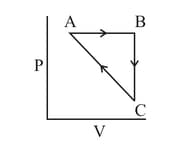
Heat absorbed by the system during process is
fish swimming in water body when taken out from the water body is covered with a film of water of weight . When it is subjected to cooking at , then the internal energy for vaporization in is integer]
[Assume steam to be an ideal gas. Given for water at and bar is ]
Under the isothermal condition, a gas at expands from to against a constant external pressure of bar. The work done by the gas is
(Given that bar)

