EASY
Earn 100
Enthalpy of fusion of hydrogen is
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Hydrogen
EASY
Oxygen (61.4%); Carbon (22.9%), Hydrogen (10.0%); and Nitrogen (2.6%). The weight which a 75kg person would gain if all atoms are replaced by atoms is:
MEDIUM
MEDIUM
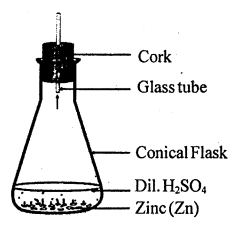
Write a chemical equation for the reaction taking place in the flask. Write name and one property of the gas evolved.
EASY
MEDIUM
Out of the oxides given below which of them cannot be reduced by ?
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
HARD
HARD
HARD
EASY
EASY

