Equal sized pieces of magnesium, strontium and calcium are placed in water. Some observations about these reactions are shown in the table. Complete the box for strontium.
Metal
Observations
Magnesium
Gives off a bubbles of gas with hot water. Dissolves very slowly.
Calcium
Gives of bubbles steadily with cold water. Dissolves slowly.
Strontium

Important Questions on Cambridge IGCSE Exam Questions from Paper 2
Complete and balance the equation for the reaction of calcium hydroxide with hydrochloric acid.
What type of chemical reaction is this?
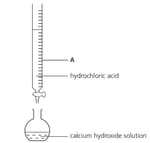
A student used the apparatus shown below to calculate the concentration of a solution of calcium hydroxide. Name the piece of apparatus labelled A.
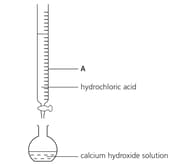
A student used the apparatus shown below to calculate the concentration of a solution of calcium hydroxide. Describe how the pH of the solution in the flask changes as the hydrochloric acid is added.
Petroleum is a mixture of hydrocarbons. It is separated into fractions such as petrol, paraffin and diesel. Name the process used to separate the fractions.
