EASY
Earn 100
Ethanoic acid reacts with sodium carbonate to form alkane.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Organic Chemistry-II
EASY
MEDIUM
EASY
Ethanoic acid was added to sodium hydrogen carbonate solution and the gas evolved was tested with a burning splinter. The following observations were reported. The correct observation is:
EASY
EASY
(a)
(b)
(c)
(d)
Brisk effervescence was observed in test tubes:
MEDIUM
If sodium carbonate is added to ethanoic acid, a gas is evolved with rapid bubbling. This gas is-
EASY
MEDIUM
In which of the following test tubes, effervescence will be observed?
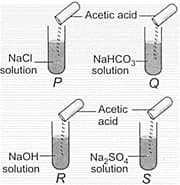
HARD
MEDIUM
MEDIUM
MEDIUM
MEDIUM
Some vinegar is dropped on solid sodium carbonate. Brisk effervescence takes place with the evolution of a colourless gas. The gas is
EASY
MEDIUM
Which of the four test tubes containing the following chemicals shows the brisk effervescence when dilute acid was added to them?
(i)
(ii)
(iii)
(iv)
EASY
HARD
Immediately a colourless and odourless gas evolves with a brisk effervescence.
The gas turns lime water milky when passed through it.
The gas burns with an explosion when a burning splinter is brought near it.
The gas extinguishes the burning splinter that is brought near it.
The correct observations are:
EASY
EASY
EASY

