EASY
Earn 100
Ethers have lower boiling points than their corresponding isomeric alcohols because of
(a)hydrogen bonding in alcohols that is absent in ethers due to low polarity
(b)hydrogen bonding in ethers due to high polarity
(c)insolubility of ethers in water due to less polarity
(d)inertness of ethers as compared to alcohols
95.65% studentsanswered this correctly
Important Questions on Alcohols, Phenols and Ethers
MEDIUM
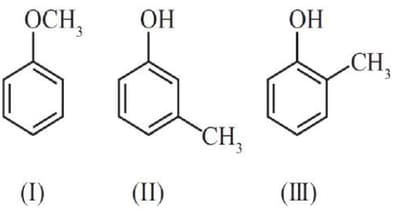
MEDIUM
Why ether is insoluble in water?
EASY
EASY
HARD
EASY
MEDIUM
HARD
MEDIUM
EASY
EASY
EASY
EASY
EASY
EASY
MEDIUM
EASY
EASY
EASY
EASY

