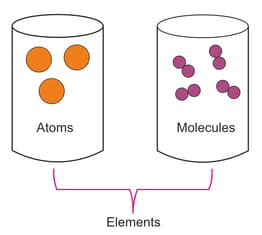
Every element is represented by its unique symbol. And symbols are used to represent the molecules of a compound as well as the molecules of elements. These molecules on the basis of the number of atoms combined in it are categorised as monatomic, diatomic or triatomic etc. You can understand the difference between atoms and molecules from the figure illustrated below. The image clearly illustrates the formation of the molecule of the elements.
Identify the compound from the following:

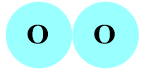


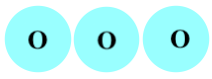

Important Questions on Atoms and Molecules
In school, the teacher taught about the law of constant proportions. A curious student thought to do the experiment and validate the law. He prepared some copper oxide from two different methods. In the first experiment, of copper oxide was obtained from of copper. In second experiment, of copper oxide gave, on oxidation, of copper. Show with the help of calculations that these figures verify the law of constant proportions.
Two elements X and Y wanted to form a compound together.
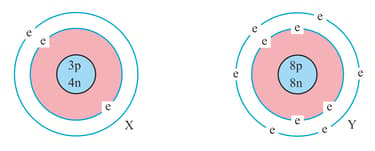
Element X said that he has the number of p = , number of e = , number of neutrons = .
Then element Y said that he has number of p = , number of e = , number of neutrons = .
The electrons in them are revolving around the nucleus in the orbits.
What are the names of the elements X and Y? When these two elements combine to form a compound, what will be the formula of the compound? Give the mass (in grams) of one mole of this compound.
mole of sodium, molecules of , atom of iron, and atoms of silver were having group discussions among themselves regarding their properties. In this discussion, one of the topics was the weight of the substances. So, they started comparing their weights to each other, but they were not able to come to any conclusion. Anand, a class 10 student, was passing from there, so they decided to take Anand's help to compare their weights.
Anand has been recently taught that for finding the mass of any element or any molecules, we should first know the atomic mass and molecular mass of the elements and molecules, respectively, and the number of moles and number of molecules of given substances. With the help of Anand, a group of students came to understand the real chemistry behind the calculation of the weight of any element or molecule. So, based on the knowledge they got from Anand, they calculated the weight of each of them.
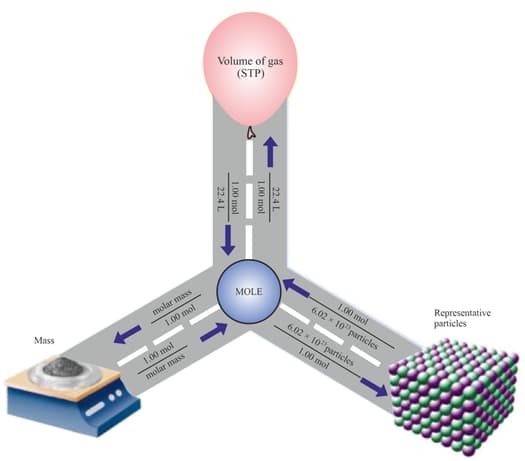
Now, can you be able to answer which of the following weighs the most?
(i) mole of sodium (ii) molecules of
(iii) atom of iron (iv) atoms of silver
(Atomic masses )
The element X is a yellow-green gas at room temperature. This element was first used in World War II to kill. It is used in drinking water and swimming pool water to kill harmful bacteria. This element, in the form of CFC, is very harmful to the ozone layer and causes a severe danger to our climate.
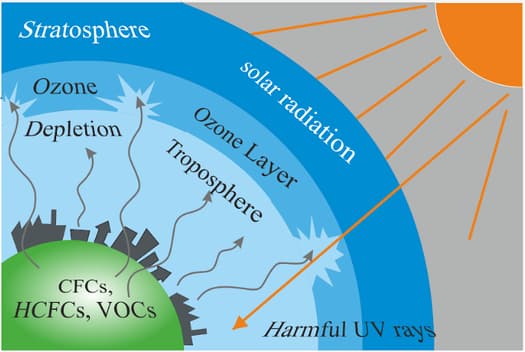
From the above explanation, can you answer the below question?
i) What is the name of the element and its atomic number?
ii) What would be the number of electrons of the element in its charge state?
We have been given element X which is very reactive and catches fire easily; hence it is stored in kerosene. Element X is very soft and can be easily cut with a knife. Element X is one of the major minerals that our body needs in relatively large amounts to stay healthy. We can find this element naturally in a variety of foods, but it is often common that we have it as the chloride of element X, also known as table salt. Element X contains three shells. The last shell contains one electron, and this electron is known as the valence electron.
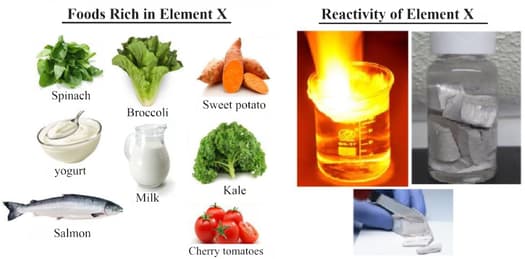
By reading the above explanation, what would element X be? What would be the nature and value of the charge on the ion formed if the valence electron was removed from the outermost shell of element X?
The chemical responsible for our identities is DNA, as it carries our genetic identity. Although it is a very complicated molecule, it consists of a sequence of just four different base units. The four base units of DNA are called adenine, guanine, cytosine and thymine. Adenine has the chemical formula , guanine has the formula , cytosine is and thymine is . The table below gives the masses of each type of atom.
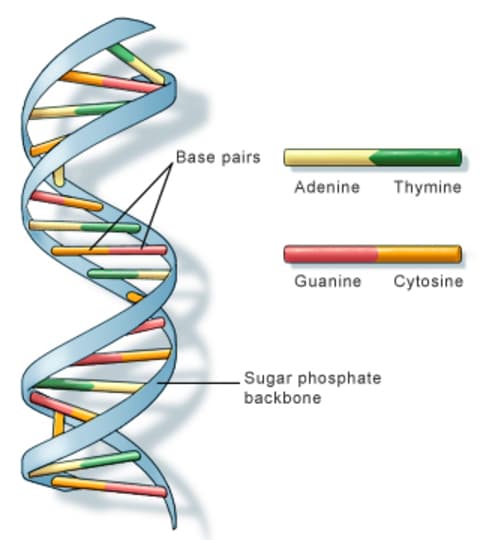
| Element | Chemical symbol | Relative atomic mass |
| hydrogen | ||
| carbon | ||
| nitrogen | ||
| oxygen | ||
| phosphorus |
- Neglecting the mass of the DNA backbone what will be the mass of the DNA with three adenine combinations and two guanine combinations.
- Calculate the number of water molecules that could be accommodated in the mass calculated in the first part.
The teacher has given one problem to Neha and Akash. She has given the formula of one compound and asked to find out the formula of another compound by using the ratio (data) of the given compound.
You can work out the formula of a compound from the ratio of different atoms in it. Sodium carbonate has the formula because it contains two atoms of sodium for every one atom of carbon and three atoms of Oxygen.
![Purchase Sodium carbonate anhydrous [497-19-8] online • Catalogo • Molekula Group](https://sss.embibe.com/cdn-cgi/image/q=75,f=auto,fit=scale-down/https://encrypted-tbn0.gstatic.com/images?q=tbn:ANd9GcRps4mH5Hp-oYGuD0x-3F0zltSkhUMOR1WRhZ_NkqkU2B8ZTUdLLKW_KIozSeTZ-cDLOZw&usqp=CAU)
Now teacher asked Neha and Akash to deduce the formula for the compound:
| Compound | Ratio in which the atoms are combined in it |
| Lead oxide |
of lead, of oxygen |
State whether the compound given, and the new compound will be ionic or covalent compounds?
Proust took two samples of copper carbonate - a compound of copper, carbon and oxygen. He took a sample from nature and another sample prepared in the lab and decomposed it chemically to find the percentage of copper, carbon and oxygen in the two samples.

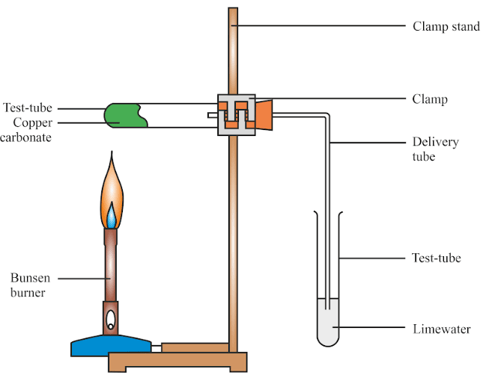
The results obtained are given in the following table.
| Element | Weight percentage | |
| Natural sample | Synthetic sample | |
| Copper | 51.35 | 51.35 |
| Carbon | 38.91 | 38.91 |
| Oxygen | 9.74 | 9.74 |
What difference do you observe in the percentage of copper, carbon and oxygen in the two samples?
