HARD
9th Kerala Board
IMPORTANT
Earn 100
Examine the illustration of the chemical bond formation in carbon tetrachloride molecule and answer the questions given below.
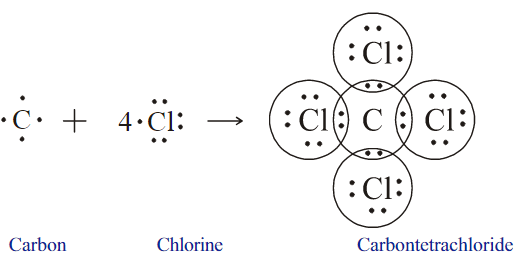
- How many pairs of electrons are shared by the carbon atom with each chlorine atom?
- What is the total number of electron pairs shared by the carbon atom with all the chlorine atoms?
- How can we represent the molecule using symbols?


Important Questions on Chemical Bonding
HARD
9th Kerala Board
IMPORTANT
Examples of some covalent compounds are given draw the chemical bonds of the compounds by using electron dot diagram.
HARD
9th Kerala Board
IMPORTANT
Some compounds and their nature are shown in the table. Complete the table by finding out the electron negativity difference between the constituent elements.
| Compounds | Difference in electronegativity of the constituent elements | Nature of the compound |
| Carbon monoxide | Covalent | |
| Sodium chloride | Ionic | |
| Methane | Covalent | |
| Magnesium chloride | Ionic | |
| Sodium oxide | Ionic |
HARD
9th Kerala Board
IMPORTANT
- What is the electronegativity of hydrogen?
- What is the electronegativity of chlorine?
- The atomic nucleus of which of these elements has a greater tendency to attract the shared pair of electrons?
HARD
9th Kerala Board
IMPORTANT
MEDIUM
9th Kerala Board
IMPORTANT
MEDIUM
9th Kerala Board
IMPORTANT
HARD
9th Kerala Board
IMPORTANT
HARD
9th Kerala Board
IMPORTANT
