Excess of calcium orthophosphate is reacted with magnesium to form calcium phosphide with magnesium oxide. Calcium phosphide on reacting with an excess of water liberate phosphine gas () along with calcium hydroxide. The evolved phosphine was burnt in the air (excess of oxygen ) to yield phosphorus pentoxide along with water. How many grams of magnesium metaphosphate would be obtained, if of magnesium were used for reducing calcium phosphate:
( Atomic wt. of )
Magnesium metaphosphate

Important Questions on Some Basic Concepts of Chemistry
Which of the following atoms/molecules are placed at the correct position?
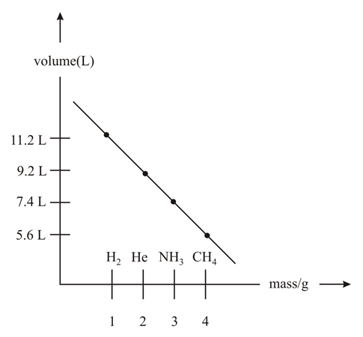
of dry green algae absorbs moles of per hour by photosynthesis. If the fixed carbon atoms were all stored after photosynthesis as starch, , how long (in hours) would it take for the algae to double their own weight assuming photosynthesis takes place at a constant rate?
Round-off your answer to two places of decimal.
