HARD
Earn 100
Explain Hinsberg test for amines.
Important Questions on Organic Compounds Containing Nitrogen
HARD
EASY
MEDIUM
Give a chemical test for primary amines.
EASY
Distinguish between the following two compounds by a chemical reaction:
and .
EASY
EASY
EASY
HARD
EASY
MEDIUM
The following pairs of compounds can be distinguished by which of the following tests:
(i) Ethylamine and aniline
(ii) Aniline and N-methylaniline
MEDIUM
MEDIUM
EASY
HARD
The following reaction constitutes :
MEDIUM
During the Hinsberg's Test, which of the following primary amine is most likely to be detected as a secondary amine?
EASY
EASY
HARD
HARD
The following reaction constitutes :
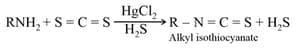
HARD

