MEDIUM
AS and A Level
IMPORTANT
Earn 100
Explain how the chains of Kevlar are held together to make it such a strong material.

Important Questions on Polymerisation
EASY
AS and A Level
IMPORTANT
Glycine is an amino acid with the formula . Give the systematic name for glycine.
EASY
AS and A Level
IMPORTANT
Glycine is an amino acid with the formula . State what type of attractive force forms between the chains of poly(glycine).
MEDIUM
AS and A Level
IMPORTANT
3-Hydroxypropanoic acid is capable of forming polymers. Give the structure of 3-hydroxypropanoic acid.
MEDIUM
AS and A Level
IMPORTANT
3-Hydroxypropanoic acid is capable of forming polymers. Give the structure of the polymer formed from this acid, showing at least two repeat units.
EASY
AS and A Level
IMPORTANT
3-Hydroxypropanoic acid is capable of forming polymers. Name the linkage present in the polymer formed from this acid.
EASY
AS and A Level
IMPORTANT
3-Hydroxypropanoic acid is capable of forming polymers. State what type of attractive force forms between chains of poly(3-hydroxypropanoic acid).
MEDIUM
AS and A Level
IMPORTANT
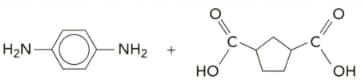
Give the structures of the polymers formed from the monomers given below, showing at least two repeat units. For each polymer identify the following:
i the repeat unit.
ii the type of linkage present.
iii the attractive force between the polymer chains.
MEDIUM
AS and A Level
IMPORTANT
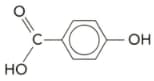
Give the structures of the polymers formed from the monomers given below, showing at least two repeat units. For each polymer identify the following:
i the repeat unit.
ii the type of linkage present.
iii the attractive force between the polymer chains.