Explain in brief the advantages and disadvantages of soft water and hard water.

Important Questions on Water
Water exhibit unusual properties in the condensed phase i.e., liquid state and solid state. This is due to the
A sample of water containing some dissolved sugar and table salt is passed through an organic ion exchange resins. The resulting water will be
Some of the mixtures are given below.
I. Air II. Sea water III. Cold drink IV. Milk
Which of the above mixtures are considered as true solutions?
Consider the following statement regarding Henry's law.
I. The solubility of solid in a liquid is directly proportional to temperature if it is endothermic reaction.
II. The solubility of a gas in a liquid is directly proportional to the partial pressure of gas present above the surface of liquid or solution.
III. The solubility of a liquid in a gas is directly proportional to the partial pressure of liquid present above the surface of gas.
IV. The solubility of a gas in solid is directly proportional to the partial pressure of gas present above the surface of solid.
Choose the correct statement (s).
The solubility of salts increases with an increase in temperature. The graph given below shows the solubility of different salts at different temperatures.
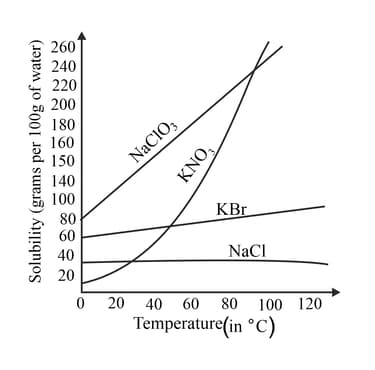
From the information given in the graph, which salt is almost equally soluble at all temperatures ?
Which one of the following graph shows solubility of a gas in a liquid?
A solution contains of common salt in of water. The concentration of the solution is
X is a hydrate of calcium. On heating, it gives an another hydrate, Y which is used for setting fractured bones. The ratio of water molecules in X and Y (X: Y) is
