MEDIUM
Earn 100
Explain the advantage of using tetramethylsilane () as the reference standard in NMR spectroscopy.
Important Questions on Measurement and Analysis
MEDIUM
Identify a correct statement about tetramethylsilane.
MEDIUM
The advantage of Tetramethyl silane as standard reference in NMR is:
MEDIUM
All the hydrogen atoms present in TMS(tetramethyl silane) are in a _____ molecular environment.(single/double)
MEDIUM
Which peak has intensity ratios of in NMR spectrum?
MEDIUM
In the low resolution spectrum of ethanol, the largest peak is generated from the hydrogens of ____.
HARD
What are the number of signals in in the given molecules?
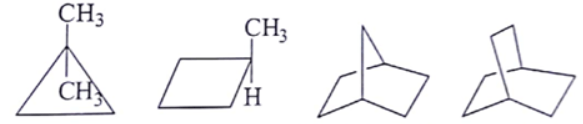
MEDIUM
Identify an incorrect statement about tetramethylsilane.
MEDIUM
Chemical shift is measured in _____(ppm/ppb)
MEDIUM
The low resolution spectrum of ethanol contains:
EASY
In H NMR stectroscopy, a triplet has an intensity ratio of _____.
MEDIUM
In a high-resolution NMR spectrum of Propanal (), the peaks are labelled as and . The triplet peak labelled corresponds to
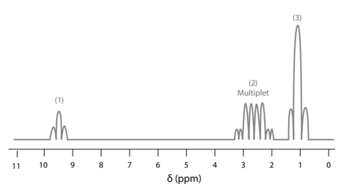
MEDIUM
According to Pascal's triangle, the intensity ratios of a triplet is
MEDIUM
Mathematically, chemical shift can be defined by
MEDIUM
The organic compound which is used as an internal standard for NMR analytical instruments is
EASY
Tetramethyl silane gives only one sharp absorption, which is called as a _____(Peak/frequency).
MEDIUM
The reference standard used in NMR spectroscopy is
MEDIUM
In spectroscopy, the splitting of single peak into many depend on the number of _____ on the neighbouring atoms.(neutrons/protons)
EASY
The frequency of nuclei relative to the standard in NMR spectrum is called _____.
MEDIUM
In the low resolution spectrum of ethanol, the smallest peak is generated from the hydrogens of ____
MEDIUM
The possible number of peaks in a high-resolution NMR spectrum of Propanal () is

