Explain the following: why are most metals strong, but ionic solids are brittle?

Important Questions on States of Matter
why is an alloy of copper and tin stronger than either copper or tin alone?
The table shows some properties of four elements. Use the data to answer the following questions. (Assume that steel has similar properties to iron.)
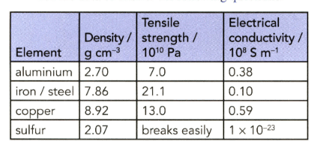
Why is aluminium with a steel core used for overhead electricity cables in preference to copper?
The table shows some properties of four elements. Use the data to answer the following questions. (Assume that steel has similar properties to iron.)
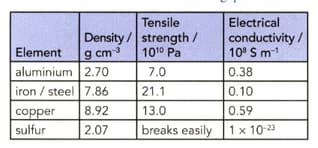
Suggest why many car engine blocks are made from aluminium alloys rather than from steel.
The table shows some properties of four elements. Use the data to answer the following questions. (Assume that steel has similar properties to iron.)
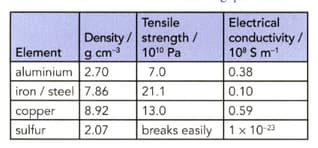
Explain the differences in tensile strength and electrical conductivity of iron and sulfur.
Explain the following property of silicon(IV) oxide by referring to its structure and bonding:
It has a high melting point.
It does not conduct electricity.
It is a crystalline solid.
silicon(IV) oxide is hard.
