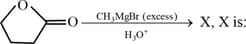EASY
Earn 100
Explain the general structure of ester.
Important Questions on Transfer
MEDIUM
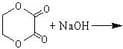
The reaction is
Write the structural formula of X.
MEDIUM
MEDIUM
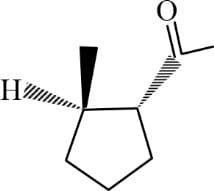
HARD
MEDIUM
EASY
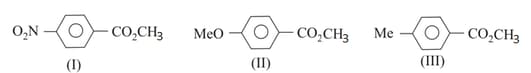
For the above three esters, the order of rates of alkaline hydrolysis is
MEDIUM
MEDIUM
The decreasing order of ease of alkaline hydrolysis for the following esters is
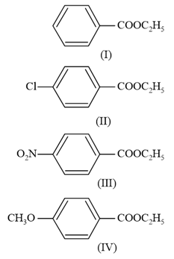
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
HARD
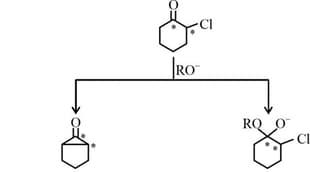
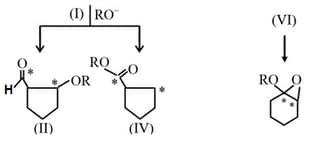
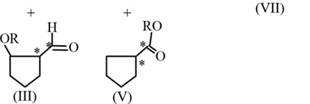
HARD
HARD
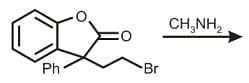
EASY
HARD


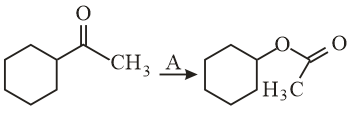 is/are
is/are