MEDIUM
Earn 100
Explain the observations made when zinc rod is kept in copper nitrate solution.
Important Questions on Redox Reactions
EASY
MEDIUM
EASY
The electrode potential, for the reduction of to in acidic medium is
V. Which of the following metal(s) will be oxidised? The reduction reactions and standard electrode potentials for and are given as
EASY
Among the following, the strongest reducing agent is:
EASY
MEDIUM
EASY
In the following resonance structures, the curved arrow indicates that electrons are shifted from
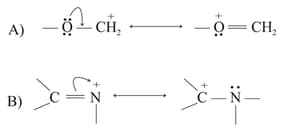
EASY
Role of hydrogen peroxide in the above reactions is respectively:
EASY
EASY
[Given : ]
(i)
(ii)
(iii)
(iv)
EASY
EASY
Which of the following statements is correct ?
EASY
MEDIUM
Given
and
MEDIUM
(Unbalanced)
HARD
HARD
The redox reaction among the following is:
MEDIUM
EASY
EASY

