Explain the shape of .

Important Questions on Chemical Bonding and Molecular Structure
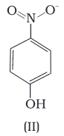
Structures of molecules of two compounds are given below :
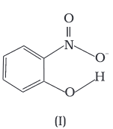
Which of the two compounds will have intermolecular hydrogen bonding and which compound is expected to show intramolecular hydrogen bonding.

Structures of molecules of two compounds are given below :
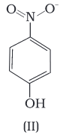
The melting point of a compound depends on, among other things, the extent of hydrogen bonding. On this basis explain which of the above two compounds will show higher melting point.
Structures of molecules of two compounds are given below :
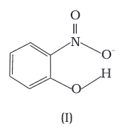
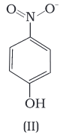
Solubility of compounds in water depends on power to form hydrogen bonds with water. Which of the above compounds will form hydrogen bond with water easily and be more soluble in it.
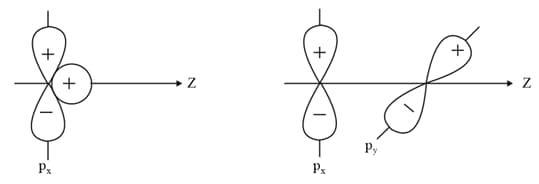
Why does type of overlap given in the following figure not result in bond formation?
Write Lewis structure of the following compounds and show formal charge on each atom.
The energy of molecular orbital is greater than and molecular orbitals in nitrogen molecule. Write the complete sequence of energy levels in the increasing order of energy in the molecule. Compare the relative stability and the magnetic behaviour of the following species :
