HARD
11th CBSE
IMPORTANT
Earn 100
Explain why ion cannot be represented by a single Lewis structure. How can it be best represented?

Important Questions on Chemical Bonding and Molecular Structure
HARD
11th CBSE
IMPORTANT
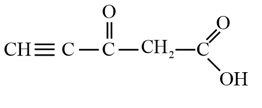
Predict the hybridisation of each carbon in the molecule of organic compound given below. Also indicate the total number of sigma and pi bonds in this molecule.
HARD
11th CBSE
IMPORTANT
Group the following as linear and non-linear molecules :
MEDIUM
11th CBSE
IMPORTANT
Elements and have and valence electrons respectively.
(i) Write the molecular formula of the compounds formed by these elements individually with hydrogen.
EASY
11th CBSE
IMPORTANT
Elements and have and valence electrons respectively.
Which of these compounds will have the highest dipole moment?
HARD
11th CBSE
IMPORTANT
Draw the resonating structure of
Ozone molecule
HARD
11th CBSE
IMPORTANT
Predict the shapes of the following molecules on the basis of hybridisation.
HARD
11th CBSE
IMPORTANT
All the bonds in carbonate ion are equal in length. Explain.
HARD
11th CBSE
IMPORTANT
What is meant by the term average bond enthalpy? Why is there a difference in bond enthalpy of bond in ethanol and water?
