Explain why the boiling points of aldehydes and ketones are lower than those of alcohol.
Important Questions on Aldehydes, Ketones and Carboxylic Acids
Given below are two statements:
Statement : The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole - dipole interactions.
Statement : The boiling points of aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of -bonding.
In the light of the above statements, choose the most appropriate answer from the options given below :
How would you account for:
Boiling points of aldehydes are lower than alcohols.
Arrange the following compounds in increasing order of their boiling points:
Arrange the following compounds in the increasing order of their boiling points.
Acetone, n-propyl alcohol, ethyl methyl ether and n-butane.
Which of the compound is soluble in ?
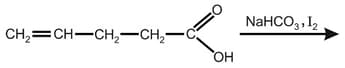
Write the following physical properties of aldehyde and ketones.
Colours and odour.
At room temperature formaldehyde is
Arrange the following compounds in the increasing order of their boiling points.
Dimethyl ether, ethyl alcohol, propane, acetaldehyde.
.

