Explain why the enthalpy change of neutralisation of one mole of sulphuric acid, is not the standard enthalpy change of neutralisation in .

Important Questions on Enthalpy Changes
A student added of sodium hydroxide to of water to make a concentrated solution. All the sodium hydroxide dissolved. The student measured the maximum temperature rise. The student suggested that these results would give an accurate value for the standard enthalpy change of solution. Give two reasons why the student is incorrect.
A student calculated the standard enthalpy change of combustion of ethanol by calorimetry as . The data book value is . Explain why there is a difference between these values.
Calculate using the following information,
Calculate a value for using the following data:
Look at this equation.
.
Which one of the following gives the correct value for the enthalpy change of this reaction?
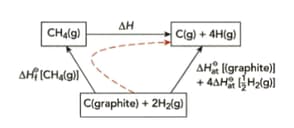
Use the information in Figure and the information below to demonstrate that the average bond energy of the bond is .