HARD
JEE Main/Advance
IMPORTANT
Earn 100
Figure shows diagram for a given mass of an ideal gas for the process During this process, density of the gas is
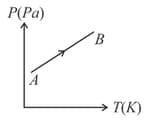

(a)Decreasing
(b)Increasing
(c)Constant
(d)First decreasing then increasing
50% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
EASY
JEE Main/Advance
IMPORTANT
Figure shows curves for a given mass of an ideal gas at pressures and Then
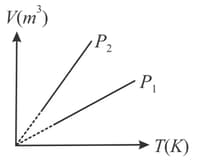
HARD
JEE Main/Advance
IMPORTANT
The diagram of a gas at constant temperature are drawn. The curve is for a constant mass and temperature and curve is for a constant mass and temperature Select the correct alternative.

EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
