EASY
JEE Main/Advance
IMPORTANT
Earn 100
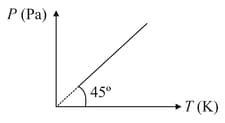
Figure shows diagram for moles of an ideal gas. The volume of the gas is nearly

(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
JEE Main/Advance
IMPORTANT
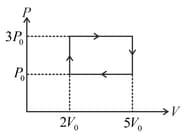
In the process shown by the graph the change in internal energy is work done by the gas is and heat absorbed by the gas is Select the correct alternatives.
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
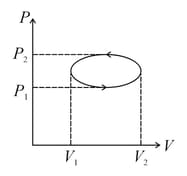
In the given elliptical diagram
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
Work done in given cyclic process is