Find out the structures of glucose, vanillin, camphor, and paracetamol. Mark the carbon atoms present in them. Assign the hybridization state to each of the carbon and oxygen atom. Identify sigma and pi bonds in these molecules.

Important Questions on Basic Principles of Organic Chemistry
Draw the structural formula of ethane.
Draw the electron-dot structure of propane.
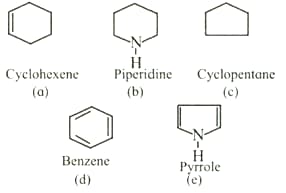
(i) Observe the compounds to
(ii) Identify the compounds those contain a ring of carbon atoms only.
(iii) Identify the compounds in which ring contains at least one atom other than carbon.
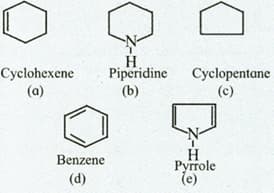
Identify the compounds those contain a ring of carbon atoms only.
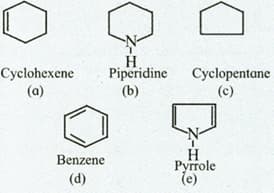
Identify the compounds in which ring contains at least one atom other than carbon.
Consider the following reaction:
Compare the structure of the substrate propanol with that of the product sodium propoxide. Which part of the substrate, the carbon skeleton, or the group has undergone a change during the reaction?
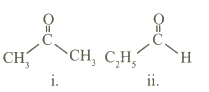
Observe the structural formulae (i) and (ii). Find out their molecular formulae. What is the difference between them? What is the relation between the two compounds represented by these structural formulae?
