HARD
Earn 100
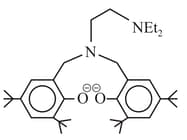
Find the denticity of the following compound
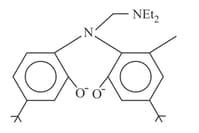
(a)2
(b)3
(c)4
(d)6
12.5% studentsanswered this correctly
Important Questions on Coordination Compounds
EASY
EASY
(en ethane-1, 2-diamine)
EASY
EASY
HARD
MEDIUM
EASY
EASY
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
EASY
The sum of coordination number and oxidation number of metal in the complex (Where en is ethylenediamine) is: