HARD
Earn 100
For a certain reaction , rate .
It is seen that for another reaction, , rate = .
Based on the above, what can be said about reactions X and Y?
(a)Both the reactions involve complex molecules.
(b)Both the reactions involve simple molecules or atomic species.
(c)Reaction involves simple molecules or atomic species, while reaction involves complex molecules.
(d)Reaction involves complex molecules, while reaction involves simple molecules or atomic species.
29.82% studentsanswered this correctly
Important Questions on Chemical Kinetics
HARD
The activation energy of the backward reaction exceeds that of the forward reaction by (in ). If the pre-exponential factor of the forward reaction is times that of the reverse reaction, the absolute value of for the reaction at is ____.
(Given; and is the Gibbs energy)
HARD
For an elementary chemical reaction, , the expression for is:
HARD
(Assume that all these gases behave as ideal gases)
HARD
MEDIUM
EASY
MEDIUM
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
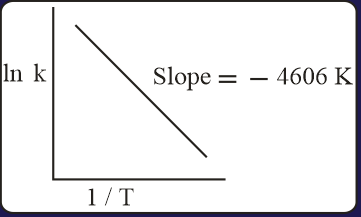
MEDIUM
[Gas constant, ]
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

EASY
EASY
Rate
If the concentration of A is kept the same but that of B is doubled what will happen to the rate itself?
HARD
EASY
EASY
MEDIUM
| Initial Concentration (A) | Initial Concentration (B) | Initial rate of formation of C |
|---|---|---|
The rate law for the formation of is
EASY
EASY
MEDIUM
Which one of the following statements is correct?
EASY
HARD

