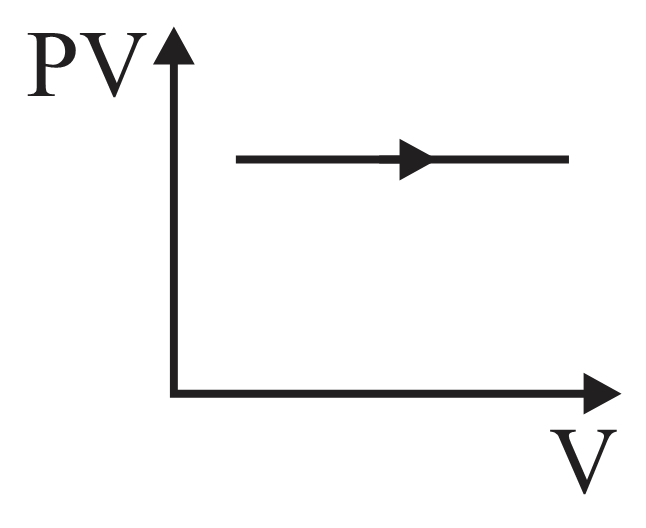
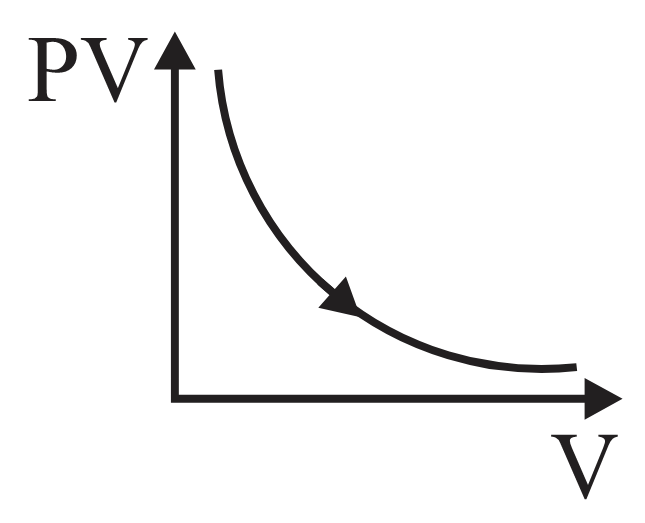
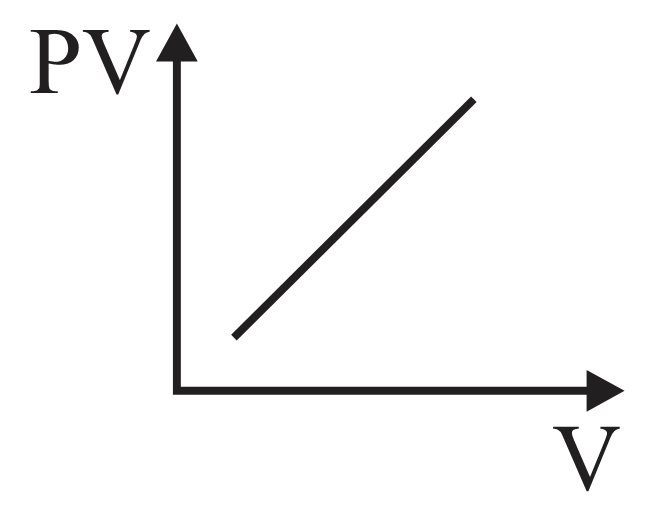
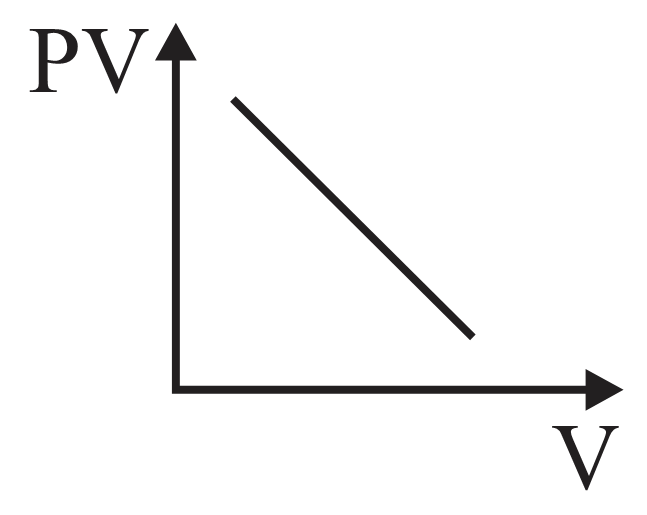
For an adiabatic process graph between and for a monatomic gas is

Important Questions on Kinetic Theory of Gases and Thermodynamics
One mole of an ideal gas is kept enclosed under a light piston (area ) connected by a compressed spring (spring constant ). The volume of gas is and its temperature is . The gas is heated so that it compresses the spring further by . The work done by the gas in the process is (Take and suppose there is no atmosphere).
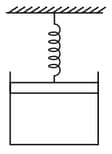
Heat energy absorbed by a system in going through a cyclic process is shown in the figure [ in litres and in ] is
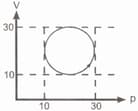
One mole of an ideal gas is taken from state to state by three different processes, (a) (b) (c) as shown in the - diagram. The heat absorbed by the gas is
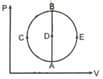
- diagram is shown below then choose the corresponding - diagram.
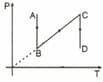
A thermodynamic process of one mole ideal monoatomic gas is shown in figure. The efficiency of cyclic process will be
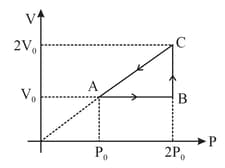
An ideal gas undergoes a thermodynamic cycle as shown in figure.
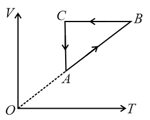
Which of the following graphs may represent the same cycle?
