EASY
JEE Main/Advance
IMPORTANT
Earn 100
For an ideal binary liquid solution with which relation between (mole fraction of A in liquid phase) and (mole fraction of A in vapour phase) is correct, and are mole fraction of B in liquid and vapour phase respectively
(a)
(b)
(c)
(d) and cannot be correlated
50% studentsanswered this correctly

Important Questions on Solutions
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
The vapour pressure of ideal solution of benzene and toluene is at then what would be correct statement about same solution at
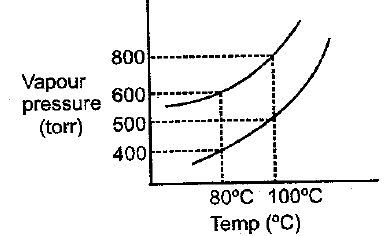
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
moles of and moles of are taken in separate beakers and enclosed in chamber Another moles of and moles of are mixed in a beaker and enclosed in chamber At equilibrium which of the following are not true. ( and are volatile liquids and they form ideal solution on mixing)
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
