MEDIUM
11th ICSE
IMPORTANT
Earn 100
For an ideal gas at constant temperature the correct graph showing variation of with is
(a)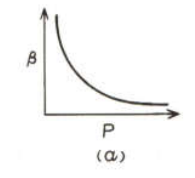

(b)

(c)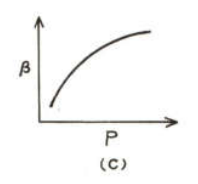

(d)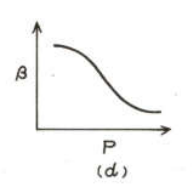

100% studentsanswered this correctly

Important Questions on Isothermal and Adiabatic Processes
MEDIUM
11th ICSE
IMPORTANT
Two samples and of a gas which are initially at the same temperature and pressure, are compressed from volume to ( isothermally and adiabatically). The final pressure of:
MEDIUM
11th ICSE
IMPORTANT
In isobaric, isothermal and adiabatic processes, for the same change in volume, work done is minimum in :
EASY
11th ICSE
IMPORTANT
Which of the following statements is true for a thermodynamic system?
EASY
11th ICSE
IMPORTANT
During the adiabatic expansion of of a gas, the change in internal energy was found to be equal to . The work done during the process will be equal to:
MEDIUM
11th ICSE
IMPORTANT
litre of helium gas at STP is adiabatically compressed to litre. Taking the initial temperature to be , the work done in the process is:
MEDIUM
11th ICSE
IMPORTANT
One kg of a diatomic gas is at a pressure of . The density of the gas is . What is the energy of the gas due to its thermal motion?
MEDIUM
11th ICSE
IMPORTANT
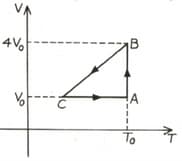
One mole of an ideal gas in initial state undergoes a cyclic process , as shown in the figure. Its pressure at A is . Choose the correct options (s) from the following: